|
Clinical Pharmacology of Marijuana
The Pharmacology of Natural Products
It is important to keep in mind that marijuana is not a single drug.
Marijuana is a mixture of the
dried flowering tops and leaves from the plant cannabis sativa (Agurell et al.
1984; Graham
1976; Jones 1987; Mechoulam 1973). Like most plants, marijuana is a variable and
complex
mixture of biologically active compounds (Agurell et al. 1986; Graham 1976;
Mechoulam 1973).
Characterizing the clinical pharmacology of the constituents in any
pharmacologically active
plant is often complicated, particularly when the plant is smoked or eaten more
or less in its
natural form. Marijuana is not unusual in this respect. Cannabis sativa is a
very adaptive plant,
so its characteristics are even more variable than most plants (Graham 1976;
Mechoulam 1973).
Some of the seeming inconsistency or uncertainty in scientific reports describing
the clinical
pharmacology of marijuana results from the inherently variable potency of the
plant material
used in research studies. Inadequate control over drug dose when researching the
effects of
smoked and oral marijuana, together with the use of research subjects who vary
greatly in their
past experience with marijuana, contribute differing accounts of what marijuana
does or does not do.
The Plant
Marijuana contains more than 400 chemicals. Approximately 60 are called
cannabinoids; i.e.,
C21 terpenes found in the plant and their carboxylic acids, analogs,
and transformation products
(Agurell et al. 1984, 1986; Mechoulam 1973). Most of the naturally occurring
cannabinoids
have been identified. Cannabinoids appear in no other plant. Cannabinoids have
been the
subject of much research, particularly since the mid 1960s when Mechoulam and his
colleagues
first isolated delta-9-tetrahydrocannabinol (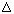 9-THC) (Mechoulam 1973;
Mechoulam et al. 1991).
THC in the scientific literature is termed
9-THC) (Mechoulam 1973;
Mechoulam et al. 1991).
THC in the scientific literature is termed 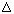 9-THC or
9-THC or 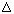 1-THC depending on whether the
pyran or
monoterpinoid numbering system is used.
1-THC depending on whether the
pyran or
monoterpinoid numbering system is used.
Cannabinoids of Importance
THC, the main psychoactive cannabinoid in marijuana, is an optically active
resinous substance.
THC is not soluble in water but is extremely lipid soluble (Agurell et al. 1984,
1986; Mechoulam
1973). Varying proportions of other cannabinoids, mainly cannabidiol (CBD) and
cannabinol
(CBN), are also present in marijuana, sometimes in quantities that might modify
the pharmacology
of THC or cause effects of their own. CBD is not psychoactive but has
significant anticonvulsant,
sedative, and other pharmacologic activity likely to interact with THC (Adams and
Martin 1996;
Agurell et al. 1984, 1986; Hollister 1986a).
The concentration of THC and other cannabinoids in marijuana varies greatly
depending on
growing conditions, plant genetics, and processing after harvest (Adams and
Martin 1996;
Agurell et al. 1984; Graham 1976; Mechoulam 1973). In the usual mixture of
leaves and stems
distributed as marijuana, concentration of THC ranges from 0.3 percent to 4
percent by weight.
However, specially grown and selected marijuana can contain 15 percent or more
THC. Thus, a
marijuana cigarette weighing 1 gram (g) might contain as little as 3 milligrams
(mg) of THC or
as much as 150 mg or more.
Potency of Tetrahydrocannabinol
THC is quite potent when compared to most other psychoactive drugs. An
intravenous (IV) dose
of only a milligram or two can produce profound mental and physiologic effects
(Agurell et al.
1984, 1986; Fehr and Kalant 1983; Jones 1987). Large doses of THC delivered by
marijuana or
administered in the pure form can produce mental and perceptual effects similar
to drugs usually
termed hallucinogens or psychomimetics. However, the way marijuana is used in
the United
States does not commonly lead to such profound mental effects. Despite potent
psychoactivity
and pharmacologic actions on multiple organ systems, cannabinoids have remarkably
low lethal
toxicity. Lethal doses in humans are not known. Given THC's potency on some
brain functions,
the clinical pharmacology of marijuana containing high concentrations of THC, for
example
greater than 10 percent, may well differ from plant material containing only 1 or
2 percent THC
simply because of the greater dose delivered.
Some Limitations of Previous Marijuana Research
Unfortunately, much of what is known about the human pharmacology of smoked
marijuana
comes from experiments with plant material containing about 2 percent THC or
less, or
occasionally up to 4 percent THC. In addition, human experiments typically are
done in
laboratory settings where only one or two smoked doses were administered to
relatively young,
medically screened, healthy male volunteers well experienced with the effects of
marijuana.
Females rarely participated in past marijuana research because of prohibitions
(now removed)
against their inclusion. Thus the clinical pharmacology of single or repeated
smoked marijuana
doses given to older people or to people with serious diseases has hardly been
researched at all in
a controlled laboratory or clinic setting. Some of the very few reports of
experiments that have
included older or sicker people, particularly patients less experienced in using
marijuana, suggest
the profile of adverse effects may differ from healthy student volunteers smoking
in a laboratory
experiment (Hollister 1986a, 1988a).
THC administered alone in its pure form is the most thoroughly researched
cannabinoid. Much
of what is written about the clinical pharmacology of marijuana is actually
inferred from the
results of experiments using only pure THC. Generally, in experiments actually
using marijuana,
the assumed dose of marijuana was based only on the concentration of THC in the
plant material.
The amounts of cannabidiol and other cannabinoids in the plant also vary so that
pharmacologic
interactions modifying the effects THC may occur when marijuana is used instead
of pure THC.
Only rarely in human experiments using marijuana was the content of CBD or other
cannabinoids
specified or the possibility of interactive effects between THC and other
cannabinoids or other
marijuana constituents actually measured.
The result of this research strategy is that a good deal is known about the
pharmacology of THC,
but experimental confirmation that the pharmacology of a marijuana cigarette is
indeed entirely
or mainly determined by the amount of THC it contains remains to be completed.
The scientific
literature contains occasional hints that the pharmacology of pure THC, although
similar, is not
always the same as the clinical pharmacology of smoked marijuana containing the
same amount
of THC (Graham 1976; Harvey 1985; Institute of Medicine 1982). Proponents of
therapeutic
applications of marijuana emphasize possible but not well documented or proven
differences
between the effects of the crude plant and pure constituents like THC (Grinspoon
and Bakalar 1993).
Route-Dependent Pharmacokinetics
Route of administration determines the pharmacokinetics of the cannabinoids in
marijuana,
particularly absorption and metabolism (Adams and Martin 1996; Agurell et al.
1984, 1986).
Typically, marijuana is smoked as a cigarette (a joint) weighing between 0.5 and
1.0 g, or in a
pipe in a way not unlike tobacco smoking. Marijuana can also be baked in foods
and eaten, or
ethanol or other extracts of plant material can be taken by mouth. Some users
claim marijuana
containing adequate THC can be heated without burning and the resulting vapor
inhaled to produce
the desired level of intoxication. This has not been studied under controlled
conditions. Pure
preparations of THC and other cannabinoids can be administered by mouth, by
rectal suppository,
by IV injection, or smoked. IV injection of crude extracts of marijuana plant
material would be
quite toxic, however.
Marijuana Smoking and Oral Administration
Smoking plant material is a special way of delivering psychoactive drugs to
the brain. Smoking
has different behavioral and physiologic consequences than oral or IV
administration. What is
well known about tobacco (nicotine) and coca (cocaine) clinical
psychopharmacology and
toxicity illustrates this point all too well. When marijuana is smoked, THC in
the form of an
aerosol in the inhaled smoke is absorbed within seconds and delivered to the
brain rapidly and
efficiently as would be expected of a very lipid-soluble drug. Peak venous blood
levels of 75 to
150 nanograms per milliliter (ng/mL) of plasma appear about the time smoking is
finished
(Agurell et al. 1984, 1986; Huestis et al. 1992a, 1992b).
Arterial concentrations of THC have
not been measured but would be expected to be much higher initially than venous
levels, as is the
case with smoked nicotine or smoked cocaine.
Oral ingestion of THC or marijuana is quite different than smoking. Maximum
THC and other
cannabinoid blood levels are only reached 1 to 3 hours after an oral dose (Adams
and Martin
1996; Agurell et al. 1984, 1986). Onset of psychoactive and other pharmacologic
effects is rapid
after smoking but much slower after oral doses.
Marijuana Smoking Behavior and Dose Control
As with any smoked drug (e.g., nicotine or cocaine), characterizing the
pharmacokinetics of THC
and other cannabinoids from smoked marijuana is a challenge (Agurell et al. 1986;
Heishman et
al. 1989; Herning et al. 1986; Heustis et al. 1992a). A person's
smoking behavior during an
experiment is difficult for a researcher to control. People differ. Smoking
behavior is not easily
quantified. An experienced marijuana smoker can titrate and regulate dose to
obtain the desired
acute psychological effects and to avoid overdose and/or minimize undesired
effects. Each puff
delivers a discrete dose of THC to the body. Puff and inhalation volume changes
with phase of
smoking, tending to be highest at the beginning and lowest at the end of smoking
a cigarette.
Some studies found frequent users to have higher puff volumes than did less
frequent marijuana
users. During smoking, as the cigarette length shortens, the concentration of
THC in the remaining
marijuana increases; thus, each successive puff contains an increasing
concentration of THC.
One consequence of this complicated process is that an experienced marijuana
smoker can regulate
almost on a puff-by-puff basis the dose of THC delivered to lungs and thence to
brain. A less
experienced smoker is more likely to overdose or underdose. Thus a marijuana
researcher attempting
to control or specify dose in a pharmacologic experiment with smoked marijuana
has only partial
control over drug dose actually delivered. Postsmoking assay of cannabinoids in
blood or urine
can partially quantify dose actually absorbed after smoking, but the analytic
procedures are
methodologically demanding, and only in recent years have they become at all
practical.
After smoking, venous blood levels of THC fall precipitously within minutes,
and an hour later
they are about 5 to 10 percent of the peak level (Agurell et al. 1986; Huestis et
al. 1992a, 1992b).
Plasma clearance of THC is quite high, 950 milliliters per minute (mL/min) or
greater; thus
approximating hepatic blood flow. However, the rapid disappearance of THC from
blood is
largely due to redistribution to other tissues in the body rather than simply
because of rapid
cannabinoid metabolism (Agurell et al. 1984, 1986). Metabolism in most tissues
is relatively
slow or absent. Slow release of THC and other cannabinoids from tissues and
subsequent
metabolism makes for a very long elimination half-time. The terminal half-life
of THC is
estimated to be from about 20 hours to as long as 10 to 13 days, though reported
estimates vary
as expected with any slowly cleared substance and the use of assays with varied
sensitivity.
Cannabinoid metabolism is extensive with at least 80 probably biologically
inactive but not
completely studied metabolites formed from THC alone (Agurell et al. 1986;
Hollister 1988a).
11-hydroxy-THC is the primary active THC metabolite. Some inactive carboxy
metabolites
have terminal half-lives of 50 hours to 6 days or more and thus serve as long
persistence markers
of prior marijuana use by urine tests. Most of the absorbed THC dose is
eliminated in feces and
about 33 percent in urine. THC enters enterohepatic circulation and undergoes
hydroxylation
and oxidation to 11-nor-9-carboxy-delta-9-THC (9-COOH-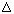 9-THC). The glucuronide is
excreted as the major urine metabolite along with about 18 nonconjugated
metabolites. Frequent
and infrequent marijuana users are similar in the way they metabolize THC
(Agurell et al. 1986;
Kelly and Jones 1992).
9-THC). The glucuronide is
excreted as the major urine metabolite along with about 18 nonconjugated
metabolites. Frequent
and infrequent marijuana users are similar in the way they metabolize THC
(Agurell et al. 1986;
Kelly and Jones 1992).
Route of Use Bioavailability and Dose
THC bioavailability, i.e., the actual absorbed dose as measured in blood, from
smoked marijuana
varies greatly among individuals. Bioavailability can range from 1 percent to 24
percent with the
fraction absorbed rarely exceeding 10 percent to 20 percent of the THC in a
marijuana cigarette
or pipe (Agurell et al. 1986; Hollister 1988a). This relatively low and
quite variable bioavailability
results from significant loss of THC in sidestream smoke, from variation in
individual smoking
behaviors, from incomplete absorption from inhaled smoke, and from metabolism in
lung and
cannabinoid pyrolysis. A smoker's experience is probably an important
determinant of dose
actually absorbed (Herning et al. 1986; Johansson et al. 1989). Much more is
known about the
dynamics of tobacco (nicotine) smoking. Many of the same pharmacokinetic
considerations
apply to marijuana smoking.
Oral bioavailability of THC, whether given in the pure form or as THC in
marijuana, also is low
and extremely variable, ranging between 5 percent and 20 percent (Agurell et al.
1984, 1986).
Great variation can occur even when the same individual is repeatedly dosed under
controlled
and ideal conditions. THC's low and variable oral bioavailability is largely a
consequence of
large first-pass hepatic elimination of THC from blood and due to erratic
absorption from stomach
and bowel. Because peak effects are slow in onset and variable in intensity,
typically at least an
hour or two after an oral dose, it is more difficult for a user to titrate dose
than with marijuana
smoking. When smoked, THC's active metabolite 11-hydroxy-THC probably
contributes little
to the effects since relatively little is formed, but after oral doses the
amounts of 11-hydroxy-THC
metabolite may exceed that of THC and thus contribute to the pharmacologic
effects of oral THC
or marijuana.
Mental and Behavioral Effects
Common Acute Effects
Usually the mental and behavioral effects of marijuana consist of a sense of
well-being (often
termed euphoria or a high), feelings of relaxation, altered perception of time
and distance,
intensified sensory experiences, laughter, talkativeness, and increased
sociability when taken in
a social setting. Impaired memory for recent events, difficulty concentrating,
dreamlike states,
impaired motor coordination, impaired driving and other psychomotor skills,
slowed reaction
time, impaired goal-directed mental activity, and altered peripheral vision are
common associated
effects (Adams and Martin 1996; Fehr and Kalant 1983; Hollister 1988a;
Institute of Medicine
1982; Tart 1971).
With repeated exposure, varying degrees of tolerance rapidly develops to many
subjective and
physiologic effects (Fehr and Kalant 1983; Jones 1987). Thus, intensity of acute
effects is
determined not only by THC dose but also by past experience, setting,
expectations, and poorly
understood individual differences in sensitivity. After a single moderate smoked
dose most
mental and behavioral effects are easily measurable for only a few hours and are
usually no
longer measurable after 4 to 6 hours (Hollister 1986a, 1988a).
A few published reports describe
lingering cognitive or behavioral changes 24 hours or so after a single smoked or
oral dose (Fehr
and Kalant 1983; Institute of Medicine 1982; Yesavage et al. 1985). Venous blood
levels of
THC or other cannabinoids correlate poorly with intensity of effects and
character of intoxication
(Agurell et al. 1986; Barnett et al. 1985; Huestis et al. 1992a).
Adverse Mental Effects
Large smoked or oral marijuana doses or even ordinary doses taken by a
sensitive, inexperienced,
or predisposed person can produce transient anxiety, panic, feelings of
depression and other
dysphoric mood changes, depersonalization, bizarre behaviors, delusions,
illusions, or hallucinations
(Adams and Martin 1996; Fehr and Kalant 1983; Hollister 1986a,
1988a; Institute of Medicine
1982). Depending on the mix of symptoms and behaviors, the state has been termed
an acute
panic reaction, toxic delirium, acute paranoid state, or acute mania. The
unpleasant effects are
usually of sudden onset, during or shortly after smoking, or appear more
gradually an hour or
two after an oral dose, usually last a few hours, less often a few days, and
completely clear
without any specific treatment other than reassurance and a supportive
environment. A subsequent
marijuana dose, particularly a lower one, may be well tolerated. In a large
survey of regular
marijuana users, 17 percent of young adult respondents reported experiencing at
least one of the
preceding symptoms during at least one occasion of marijuana use, usually early
in their use
(Tart 1971).
Whether marijuana can produce or trigger lasting mood disorders (depression or
mania) or
schizophrenia is less clearly established (Fehr and Kalant 1983; Gruber and Pope
1994; Hollister
1986a, 1988a; Institute of Medicine 1982). A psychotic state
with schizophrenic-like and manic
features lasting a week or more has been described. Marijuana can clearly worsen
schizophrenia.
Chronic marijuana use can be associated with behavior characterized by apathy and
loss of
motivation along with impaired educational performance even without obvious
behavioral
changes (Pope and Yurgelun-Todd 1996; Pope et al. 1995). The explanation and
mechanisms for
this association are still not well established.
Cardiovascular and Autonomic Effects
A consistent, prominent, and sudden effect of marijuana is a 20 to 100 percent
increase in heart
rate lasting up to 2 to 3 hours (Hollister 1986a, 1988a; Jones
1985). After higher smoked or oral
doses postural hypotension and associated faintness or dizziness can occur upon
standing up
from a supine or prone position. Tolerance to these effects appears after only a
few days of two
to three times per day dosing (Benowitz and Jones 1981; Jones 1985). Typical is
a modest
increase in supine blood pressure. Cardiac output can increase 30 percent when
supine. Peripheral
vascular resistance decreases with the greatest drop in resistance in skeletal
muscles. Skin
temperature drops are large; 4 to 6 degrees centigrade, even after a modest
smoked dose and
roughly parallel to plasma norepinephrine increases. With a few days of repeated
exposure to
frequent doses of oral THC or marijuana extract, supine blood pressure falls, the
sometimes
marked initial orthostatic hypotension disappears, blood volume increases, and
heart rate slows
(Benowitz and Jones 1981). Thus like other system effects, the intensity and
character of many
hemodynamic effects of single smoked doses in humans are a function of recent
marijuana
exposure, dose, and even body position.
The cardiovascular effects of smoked or oral marijuana have not presented any
health problems
for healthy and relatively young users. However, marijuana smoking by older
patients, particularly
those with some degree of coronary artery or cerebrovascular disease, is likely
to pose greater
risks because of the resulting increased cardiac work, increased catecholamines,
carboxyhemoglobin,
and postural hypotension (Benowitz and Jones 1981; Hollister 1988a).
Such issues have not
been well addressed in past marijuana research.
Respiratory System Effects
Pulmonary effects associated with marijuana smoking include transient
bronchodilation after
acute exposure. Chronic bronchitis and pharyngitis are associated with repeated
exposure with
an increased frequency of pulmonary illness. With chronic marijuana smoking,
large-airway
obstruction is evident on pulmonary function tests, and cellular inflammatory
histopathological
abnormalities appear in bronchial epithelium (Adams and Martin 1996; Hollister
1986a). These
effects appear to be additive to those produced by tobacco smoking.
Endocrine System
Endocrine system effects include a moderate depression of spermatogenesis and
sperm motility
and a decrease in plasma testosterone in males. Prolactin, FSH, LH, and GH
levels are decreased
in females. Although suppressed ovulation and other ovulatory cycle changes
occur in nonhuman
primates, a study of human females smoking marijuana in a research hospital
setting did not find
hormone or menstrual cycle changes like those in the monkeys given THC (Mendelson
and
Mello 1984; Mendelson et al. 1984a). Relatively little research has
been done on experimentally
administered marijuana effects on human female endocrine and reproductive system
function.
Immune System
THC and other cannabinoids in marijuana have immunosuppressant properties
producing impaired
cell-mediated and humoral immune system responses. A large literature describes
the results of
experiments with animal and animal tissue in in vivo and in vitro model systems.
THC and other
cannabinoids suppress antibody formation, cytokine production, leukocyte
migration and natural
killer-cell activity. Cannabinoids decrease host resistance to infection from
bacterial and viral
infection in animals. Marijuana smokers show evidence of impaired immune
function: for
example, decreased leukocyte blastogenesis in response to mitogens. Marijuana
smokers, when
compared to nonmarijuana smokers, have more respiratory illness (Polen et al.
1993).
The cannabinoids have been characterized as immunomodulators because although
they generally
suppress, they occasionally enhance some immune responses (Friedman et al. 1995).
Reviews of
marijuana immune system effects have characterized the effects as complicated or
conflicting or
controversial (Adams and Martin 1996; Hollister 1988b). The clinical
significance or relevance
of these findings remains uncertain. Much of the complexity and controversy
results from the
use of mostly in vitro animal models, or in vitro animal and human cell cultures,
or in vivo
animal studies. Generally in most studies the cannabinoid doses or concentrations
used have been
quite high when compared to reasonable levels of exposure in human marijuana
smoking.
Suppressed or impaired immune mechanisms would likely have negative effects on
health by
increasing susceptibility to infection or to tumors. People with compromised
immune systems or
existing malignancies may be at higher risk than healthy people. For example,
the risk of developing
AIDS may be higher with HIV infection, with a higher risk for infection by
opportunistic bacteria,
fungi, or viruses. On the other hand, some have suggested that the
immunosuppressive effects of
cannabinoids might be useful clinically; for example, in treating multiple
sclerosis, mostly
reasoning from theoretical assumptions or experimental disease models in animals.
In summary, there is good evidence that THC and other cannabinoids can impair
both cell-mediated
and humoral immune system functioning, leading to decreased resistance to
infection by viruses
and bacteria. However, the health relevance of these findings to human marijuana
use remains
uncertain. Conclusive evidence for increased malignancy, or enhanced acquisition
of HIV, or the
development of AIDS, has not been associated with marijuana use.
There is a need for further research, particularly in circumstances where
long-term administration
of marijuana might be considered for therapeutic purposes; for example, in
individuals who are
HIV-positive or who have tumors, malignancies, or diseases where immune system
function may
be important in the genesis of the disease. Clinical studies with smoked
marijuana in patients
with compromised immune systems may offer a sensitive index of adverse immune
system
effects associated with cannabinoid exposure. Direct measures of viral load and
other sensitive
indices of immune system function are now more practical than in past years when
most of the
cannabinoid immune system research was carried out. The possibility that
frequent and prolonged
marijuana use might lead to clinically significant impairments of immune system
function is
great enough that such studies should be part of any marijuana medication
development research,
particularly when marijuana will be used by patients with compromised immune
systems.
Tolerance and Physical Dependence
After repeated smoked or oral marijuana doses, marked tolerance is rapidly
acquired (after a day
or two) to many marijuana effects, e.g., cardiovascular, autonomic, and many
subjective effects.
After exposure is stopped, tolerance is lost with similar rapidity (Jones et al.
1981). Measurable
tolerance or tachyphalaxis is evident for some hours after smoking even a single
marijuana cigarette.
Withdrawal symptoms and signs appearing within hours after cessation of
repeated marijuana
use have been occasionally reported by patients in clinical settings (Duffy and
Milin 1996;
Mendelson et al. 1984b). A withdrawal syndrome was reliably produced by
as little as 5 days of
modest but frequent oral doses of THC or marijuana extract in double-blind,
placebo-controlled
experiments (Jones et al. 1981). THC decreased or relieved the symptoms.
Typical symptoms
and signs were restlessness, insomnia, irritability, salivation, tearing, nausea,
diarrhea, increased
body temperature, anorexia, weight loss, tremor, sweating, sleep brainwave rapid
eye movement
rebound, and subjective sleep disturbance. Increased dreaming contributing to
the sleep disturbance
sometimes persisted for weeks, but the other signs and symptoms were gone or
markedly
diminished within 48 hours after the last oral marijuana dose.
Drug Interactions With Marijuana
Tobacco, ethanol, and other psychoactive and therapeutic drugs commonly
consumed together
with marijuana share metabolic pathways with cannabinoids, so metabolic
interactions are likely.
Both THC and CBD inhibit the metabolism of drugs metabolized by hepatic
mixed-function
oxidase enzymes (Benowitz and Jones 1977; Benowitz et al. 1980; Hollister
1986b).
The absorption or clearance of other drugs taken with marijuana may be slowed
or hastened
depending on timing and sequence of drug ingestion and past exposure. For
example, ethanol
consumed just after smoking a marijuana cigarette produces a much lower peak
blood level than
the same dose of ethanol taken an hour before marijuana smoking because THC slows
gastric
emptying time, thus slowing absorption of ethanol.
THC is highly bound to plasma proteins (97 percent to 99 percent) and thus is
likely to interact
with other highly bound drugs because of competition for binding sites on plasma
proteins.
Finally, there is experimental evidence for drug interactions at the
functional (neural) adaptation
level (Adams and Martin 1996).
By those and possibly by other mechanisms, recent or concurrent THC or CBD
exposure
measurably alters the pharmacokinetics and/or effects of ethanol, barbiturates,
nicotine,
amphetamines, cocaine, phencyclidine, opiates, atropine, and clomipramine (Fehr
and Kalant
1983; Institute of Medicine 1982). Marijuana use is likely to alter the
pharmacology of some
concurrently used therapeutic drugs, e.g., cancer chemotherapeutic agents or
anticonvulsants.
Cannabinoid Receptors
Mechanisms of psychoactive cannabinoid action were long suspected to be
through interactions
of/with lipid components of cell membranes (Adams and Martin 1996; Hollister
1988a). The
discovery of cannabinoid receptors in the human brain in the late 1980s led to
renewed interest in
the pharmacology and potential therapeutic uses of cannabinoids (Adams and Martin
1996;
Herkenham 1992). The mechanisms of action of THC are now assumed to be mainly
receptor
mediated. So far, it still is a relatively simple receptor family (CB 1 and CB
2). Receptors are
abundant in brain areas concerned with memory, cognition, and motor coordination.
An endogenous
ligand, a fatty acid derivative named anandamide, has been identified but not yet
studied in
humans (Thomas et al. 1996). A specific THC antagonist, SR141716A, provokes
intense
withdrawal signs and behaviors in rodents that have been exposed to THC for even
relatively
brief periods (Adams and Martin 1996). The clinical pharmacology of the
antagonist has not
been studied in humans.
References
Adams, I.B., and Martin, B.R. Cannabis: Pharmacology and toxicology in animals and humans. Addiction 91(11):1585-1614, November 1996.
Agurell, S., Dewey, W.L., and Willett, R.E., eds. The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. New York: Academic Press, 1984.
Agurell, S.; Halldin, M.; Lindgren, J.E.; Ohlsson, A.; Widman, M.; Gillespie, H.; and Hollister, L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 38(1):21-43, March 1986.
Barnett, G.; Licko, V.; and Thompson, T. Behavioral pharmacokinetics of marijuana. Psychopharmacology 85(1):51-56, 1985.
Benowitz, N.L., and Jones, R.T. Effect of delta-9-tetrahydrocannabinol on drug distribution and metabolism: Antipyrine, pentobarbital and ethanol. Clin Pharmacol Ther 22(3):259-268, 1977.
Benowitz, N.L., and Jones, R.T. Cardiovascular and metabolic considerations in prolonged cannabinoid administration in man. J Clin Pharmacol 21:214S-223S, 1981.
Benowitz, N.L.; Nguyen, T.; Jones, R.T.; Herning, R.I.; and Bachman, J. Metabolic and psychophysiologic studies of cannabidiol-hexobarbital interaction. Clin Pharmacol Ther 28:115-120, 1980.
Duffy, A., and Milin, R. Case study: Withdrawal syndrome in adolescent chronic cannabis users. J Am Acad Child Adolesc Psychiatry 35(12):1618-1621, December 1996.
Fehr, K., and Kalant, H., eds. ARF/WHO Scientific Meeting on Adverse Health and Behavioral Consequences of Cannabis Use (1981: Toronto, Canada) Cannabis and Health Hazards: Proceedings of an ARF/WHO Scientific Meeting on Adverse Health and Behavioral Consequences of Cannabis Use. Toronto, Canada: Addiction Research Foundation, 1983.
Friedman, H.; Klein, T.W.; Newton, C.; and Daaka, Y. Marijuana, receptors and immunomodulation. Adv Exp Med Biol 373:103-113, 1995.
Graham, J.D.P., ed. Cannabis and Health. New York: Academic Press, 1976.
Grinspoon, L., and Bakalar, J.B. Marihuana, the Forbidden Medicine. New Haven: Yale University Press, 1993.
Gruber, A.J., and Pope, H.G. Cannabis psychotic disorder: Does it exist? Am J Addict v3 (n1):72-83, Winter 1994.
Harvey, D.J., ed. Satellite Symposium on Cannabis (3rd: 1984: Oxford, England) Marihuana '84: Proceedings of the Oxford Symposium on Cannabis. Washington, DC: IRL Press, 1985.
Heishman, S.J.; Stitzer, M.L.; and Yingling, J.E. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol Biochem Behav 34(1):173-179, September 1989.
Herkenham, M. Cannabinoid receptor localization in brain: Relationship to motor and reward systems. In: Kalivas, P.W., and Samson, H.H., eds. The neurobiology of drug and alcohol addiction. Ann N Y Acad Sci 654:19-32, 1992.
Herning, R.I.; Hooker, W.D.; and Jones, R.T. Tetrahydrocannabinol content and differences in marijuana smoking behavior. Psychopharmacology 90(2):160-162, 1986.
Hollister, L.E. Health aspects of cannabis. Pharmacol Rev 38(1):1-20, March 1986a.
Hollister, L.E. Interactions of cannabis with other drugs in man. In: Braude, M.C., and Ginzburg, H.M., eds. Strategies for Research on the Interactions of Drugs of Abuse. National Institute on Drug Abuse Research Monograph 68. DHHS Pub. No. (ADM)86-1453. Washington, DC: Supt. of Docs., U.S. Govt. Print. Off., 1986b. pp. 110-116.
Hollister, L.E. Cannabis--1988. (Literature review). Acta Psychiatr Scand (Suppl) 78(345):108-118, 1988a.
Hollister, L.E. Marijuana and immunity. J Psychoactive Drugs 20(1:):3-8, January-March 1988b.
Huestis, M.A.; Henningfield, J.E.; and Cone, E.J. Blood Cannabinoids. 1. Absorption of THC and formation of 11-OH-THC and THC COOH during and after smoking marijuana. J Anal Toxicol 16(5):276-282, September-October 1992a.
Huestis, M.A.; Sampson, A.H.; Holicky, B.J.; Henningfield, J.E.; et al. Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther 52 (1):31-41, July 1992b.
Institute of Medicine. Division of Health Sciences Policy. Marijuana and Health: Report of a Study by a Committee of the Institute of Medicine, Division of Health Sciences Policy. Washington, DC: National Academy Press, 1982.
Johansson, E.; Halldin, M.M.; Agurell, S.; Hollister, L.E.; and Gillespie, H.K. Terminal elimination plasma half-life of delta 1-tetrahydrocannabinol (delta 1-THC) in heavy users of marijuana. Eur J Clin Pharmacol 37(3):273-277, 1989.
Jones, R.T. Drug of abuse profile: Cannabis. Clin Chem 33 (11 Suppl):72B-81B, October 1987.
Jones, R.T. Cardiovascular effects of cannabinoids. In: Harvey, D.J., ed. Marihuana, '84: Proceedings of the Oxford Symposium on Cannabis. Oxford: IRL Press, 1985. pp. 325-334.
Jones, R.T.; Benowitz, N.L.; and Herning, R.I. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol 21:143S-152S, 1981.
Kelly, P., and Jones, R.T. Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. J Anal Toxicol 16:228-235, 1992.
Mechoulam, R., ed. Marijuana: Chemistry, Pharmacology, Metabolism and Clinical Effects. New York: Academic Press, 1973.
Mechoulam, R.; Devane, W.A.; Breuer, A.; and Zahalka, J. A random walk through a cannabis field. Special Issue: Pharmacological, chemical, biochemical and behavioral research on cannabis and the cannabinoids. Pharmacol Biochem Behav 40(3):461-464, November 1991.
Mendelson, J.H., and Mello, N.K. Effects of marijuana on neuroendocrine hormones in human males and females. In: Braude, M.C., and Ludford, J.P., eds. Marijuana Effects on the Endocrine and Reproductive Systems. National Institute on Drug Abuse Research Monograph 44. DHHS Pub. No. (ADM)84-1278. Washington, DC: Supt. of Docs., U.S. Govt. Print. Off., 1984. pp. 97-114.
Mendelson, J.H.; Mello, N.K.; Cristofaro, P.; Ellingboe, J.; and Benedikt, R. Acute effects of marijuana on pituitary and gonadal hormones during the periovulatory phase of the menstrual cycle. In: Harris, L.S., ed. Problems of Drug Dependence, 1984: Proceedings of the 46th Annual Scientific Meeting, The Committee on Problems of Drug Dependence, Inc. National Institute on Drug Abuse Research Monograph 55. DHHS Pub. No. (ADM)85-1393. Washington, DC: Supt. of Docs., U.S. Govt. Print. Off., 1984a. pp. 24-31.
Mendelson, J.H.; Mello, N.K.; Lex, B.W.; and Bavli, S. Marijuana withdrawal syndrome in a woman. Am J Psychiatry 141(10):1289-1290, October 1984b.
Polen, M.R.; Sidney, S.; Tekawa, I.S.; Sadler, M.; and Friedman, G.D. Health care use by frequent marijuana smokers who do not smoke tobacco. West J Med 158(6):596-601, June 1993.
Pope, H.G., Jr., and Yurgelun-Todd, D. The residual cognitive effects of heavy marijuana use in college students. JAMA 275(7):521-527, February 21, 1996.
Pope, H.G.; Gruber, A.J.; and Yurgelun-Todd, D. The residual neuropsychological effects of cannabis: The current status of research. Drug Alcohol Depend 38(1):25-34, April 1995.
Tart, C.T. On Being Stoned: A Psychological Study of Marijuana Intoxication. Palo Alto, CA: Science and Behavior Books, 1971.
Thomas, B.F.; Adams, I.B.; Mascarella, S.W.; Martin, B.R.; and Razdan, R.K. Structure-activity analysis of anandamide analogs: Relationship to a cannabinoid pharmacophore. J Med Chem 39(2):471-497, January 19, 1996.
Yesavage, J.A.; Leirer, V.O.; Denari, M.; and Hollister, L.E. Carry-over effects of marijuana intoxication on aircraft pilot performance: A preliminary report. Am J Psychiatry 142(11):1325-1329, November 1985.
Go to the Medical Marijuana Menu
Go to the HIV & Nutrition Menu
Go to the HIVpositive.us Main Menu